Dr. Grover P. Miller Ph.D. and Dr. S. Joshua Swamidass M.D. Ph.D. are pleased to announce that our grant “Computationally Modeling the Impact of Ontogeny on Drug Metabolic Fate” was funded by the National Library of Medicine (NLM) of the NIH after receiving an outstanding impact score of 20 (6th percentile) by the independent study section.
This grant extends our other recently awarded grant into modeling drug metabolism into children. Dr. Swamidass’ group will use mathematical models to understand how children metabolize drugs differently than adults. Dr. Miller’s group will validate these models with prospect experiments. Understanding these differences, we hope, will help researchers develop safer and more effective medicines for children.
In addition to thanking the NLM and our department chairman for their ongoing support, we acknowledge Na Le Dang (MD PhD student), Tyler Hughes (PhD student), Matthew Matlock (MD PhD student), and Jed Zaretzki (Postdoctoral Fellow). These brilliant and hardworking young scientists did the groundbreaking work (here, here, here, here) that underlies this project.
Public Health Relevance:
Robust strategies are needed to optimize drug dosages and minimize toxicity risks for children. Computational models are attractive and practical tools for predicting drug metabolism, yet current models can only predict factors that impact metabolism and not the process itself. Our approach explicitly models the impact of age on metabolism to improve knowledge of drug activity and clearance along with insights on age-dependent formation of toxic metabolites. This information could be leveraged to further refine models for safely dosing children.
Abstract:
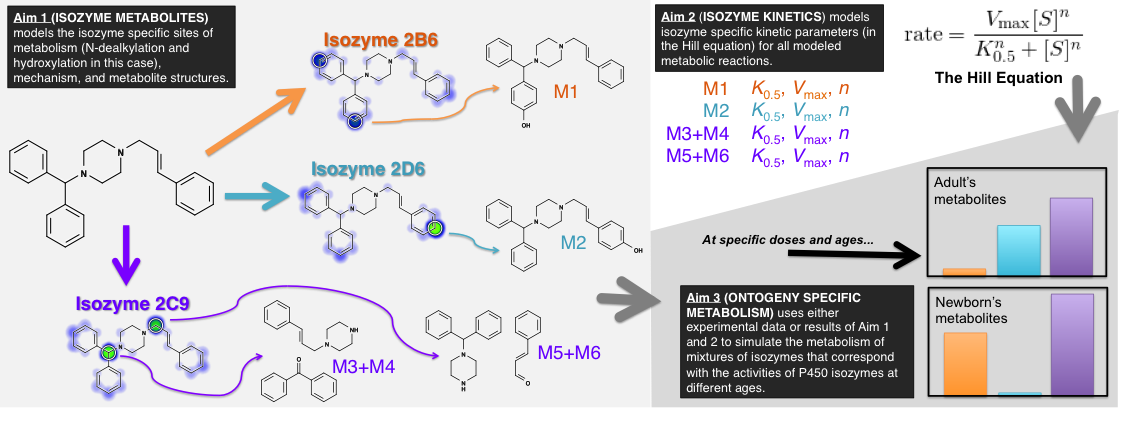
Advancements in pediatric drug development require innovative approaches that overcome challenges in assessing age-dependent drug disposition and toxic risks related to metabolism. Cytochromes P450 dominate drug metabolism yet roles for individual enzymes depend on genetics, disease states, co-medications, and ontogeny. Failure to account for those differences contributes to dosing challenges and possibly toxicity as reported for dextromethorphan, midazolam, and phenytoin. The NICHD Pediatric Formulation Initiative (PFI) workshops emphasized the need for better modeling to describe and predict how drug metabolism changes for children. Current pharmacokinetic models predict how drug clearance changes with age so as to affect the optimal dose, but those models are limited in two ways; (1) they require experimentally determined kinetic data for several enzymes that is not often available, and (2) they do not model formation of specific drug metabolites, which is important in predicting toxicity and drug interactions, regardless of whether clearance changes with age. We hypothesize that computational models of mixtures of P450 enzymes can predict how the “metabolic fate” of drugs changes with age, i.e. the kinetics of drug metabolism and the resulting metabolite structures. We propose building hierarchical mathematical models that at first predict the drug metabolites formed by metabolic enzymes (Aim 1), then the efficiency of formation for each metabolite (the kinetics) (Aim 2), and combine these models to predict metabolites formed by age-specific mixtures of P450s (Aim 3). This proposal makes significant steps toward achieving PFI goals. First, datasets created for this study will be made publicly available to foster model refinement and validation by the community. Second, simulation of metabolite profiles would yield tractable biomarkers and support studies on possible age-dependent drug-drug interactions, off-target biological activities, pro-drug activation, and formation of toxic species. Third, the models will indicate, for both new and existing drugs, when metabolic fate (consequently, toxicity and interactions) changes in pediatric patients, even when pharmacokinetics stay the same. Fourth, though not the primary aim of this study, successfully modeling metabolic efficiency could enable pharmacokinetic studies for predicting problematic pediatric drugs prior to carrying out any necessary experimental kinetic studies. Taken together, this proposal lays a strong foundation for developing models relevant that resolve challenges in optimizing drug dosages and minimizing toxicity risks for children.

You must log in to post a comment.