A Computational Approach to Structural Alerts: Furans, Phenols, Nitroaromatics, and Thiophenes
Dang, N. L., Hughes, T. B., Miller, G. P., and Swamidass, S. J. (2017). Chemical Research in Toxicology, DOI: 10.1021/acs.chemrestox.6b00336
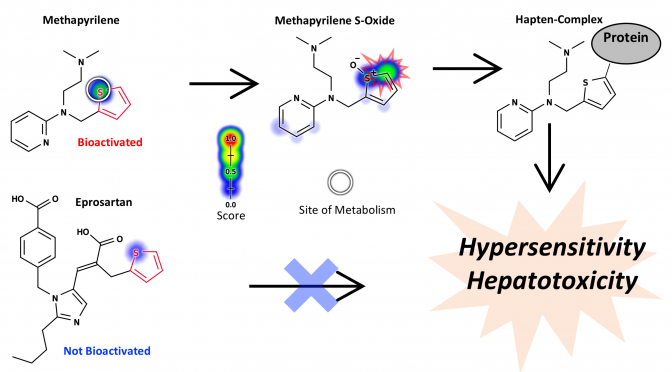
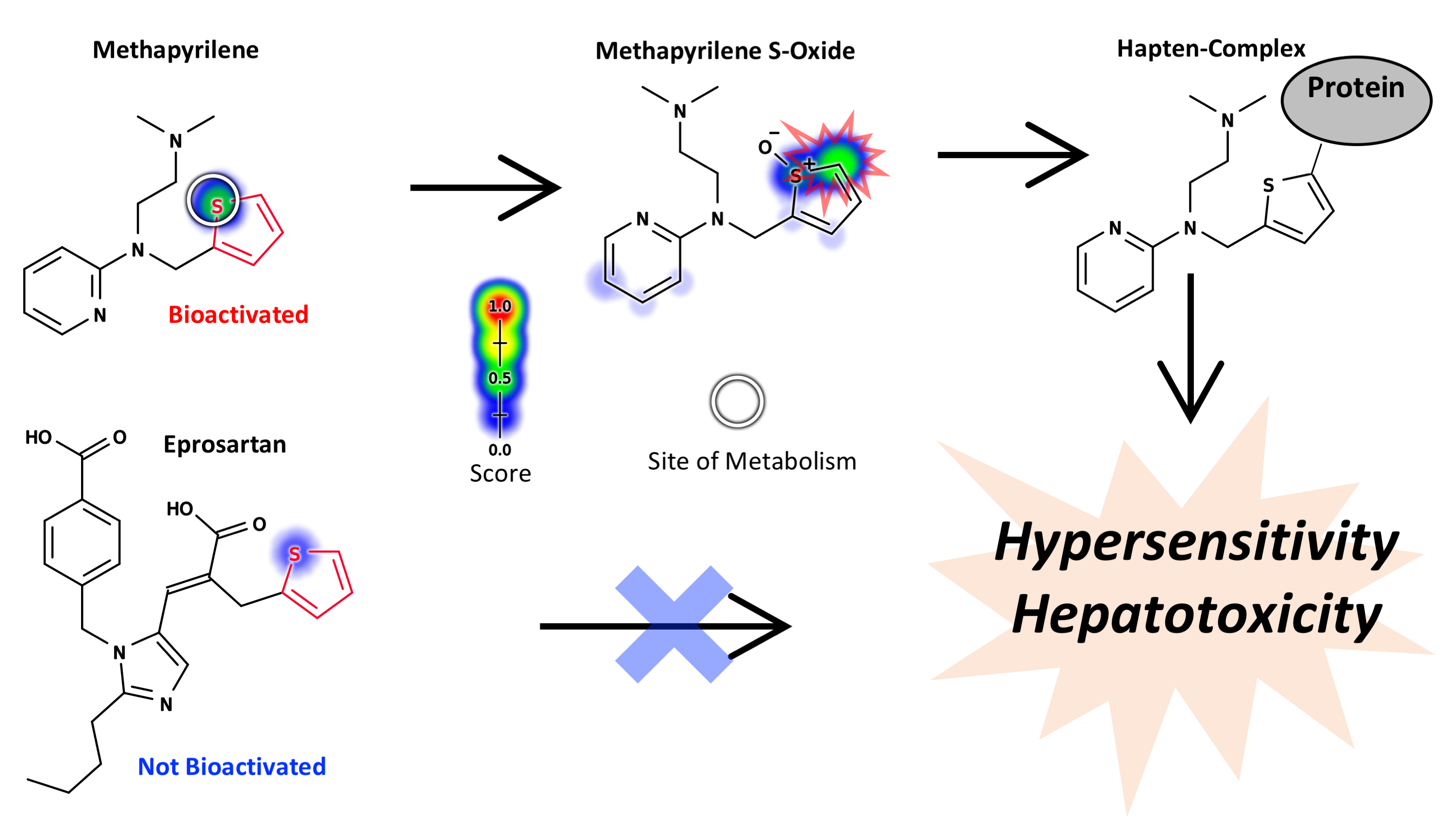
Abstract: Structural alerts are commonly used in drug discovery to identify molecules likely to form reactive metabolites, and thereby become toxic. Unfortunately, as useful as structural alerts are, they do not effectively model if, when, and why metabolism renders safe molecules toxic. Toxicity due to a specific structural alert is highly conditional, depending on the metabolism of the alert, the reactivity of its metabolites, dosage, and competing detoxification pathways. A systems approach, which explicitly models these pathways, could more effectively assess the toxicity risk of drug candidates. In this study, we demonstrated that mathematical models of P450 metabolism can predict the context-specific probability that a structural alert will be bioactivated in a given molecule. This study focuses on the furan, phenol, nitroaromatic, and thiophene alerts. Each of these structural alerts can produce reactive metabolites through certain metabolic pathways, but not always. We tested whether our metabolism modeling approach, XenoSite, can predict when a given molecule’s alerts will be bioactivated. Specifically, we used models of epoxidation, quinone formation, reduction, and sulfur-oxidation to predict the bioactivation of furan-, phenol-, nitroaromatic-, and thiophene-containing drugs. Our models separated bioactivated and not-bioactivated furan-, phenol-, nitroaromatic-, and thiophene-containing drugs with AUC performances of 100%, 73%, 93%, and 88%, respectively. Metabolism models accurately predict whether alerts are bioactivated and thus serve as a practical approach to improve the interpretability and usefulness of structural alerts. We expect that this same computational approach can be extended to most other structural alerts and later integrated into toxicity risk models. This advance is one necessary step towards our long-term goal of building comprehensive metabolic models of bioactivation and detoxification to guide assessment and design of new therapeutic molecules.
Publication: http://pubs.acs.org/doi/abs/10.1021/acs.chemrestox.6b00336

You must log in to post a comment.