Dr. Grover P. Miller Ph.D. and Dr. S. Joshua Swamidass M.D. Ph.D. are pleased to announce that our grant “Data and Tools for Modeling Metabolism and Reactivity” was funded by the National Library of Medicine (NLM) of the NIH after receiving an outstanding impact score of 21 (5th percentile) by the independent study section.
This grant will fund research in our collaboration over the next several years. Dr. Swamidass’ group will use mathematical models to understand how and when “reactive metabolites” are formed, and validate these models with prospective experiments in Dr. Miller’s group. Reactive metabolites are the root cause of some of the most difficult to predict adverse drug reactions.
In addition to thanking the NLM and our department chairman for their ongoing support, we acknowledge Na Le Dang (MD PhD student), Tyler Hughes (PhD student), Matthew Matlock (MD PhD student), and Jed Zaretzki (Postdoctoral Fellow). These brilliant and hardworking young scientists did the groundbreaking work (here, here, here, here) that underlies this project.
Opportunities to Participate:
In the coming weeks, we will be releasing more information about how members of the scientific community can collaborate and benefit from our work.
- Modeling experts are invited to ensure our data curation efforts will become a standard benchmark in the field. All data will be available for free for non-commercial purposes.
- Academic drug development groups are invited to work with us to identify and validate bioactivation pathways that could cause their drug candidates to be toxic. Our tools will be released as XenoSite extensions on our website.
- Industry drug development groups are invited to apply our work with in house software, currently available over the Amazon cloud to securely protect the privacy of proprietary chemical information.
- Metabolism researchers are invited to participate with us by using their ongoing studies in metabolism and reactivity to prospectively test our approach, and contribute structured data to the scientific modeling community.
More details on these opportunities to participate will be released regularly over the coming months.
Public Health Relevance:
Adverse drug reactions, especially rare but severe hypersensitivity driven reactions, have profound financial and health implications and are caused by reactive drug metabolites. As an extension of tools and data developed by our group, we will build and test mathematical models and datasets that more accurately predict when molecules form clinically important reactive metabolites than current methodologies, such as “structural alerts.” We will use these models to build a tool that will better predict when drug candidates form reactive metabolites, which will make new medicines safer by enabling researchers to avoid drug candidates prone to causing adverse reactions.
Abbreviated Abstract:
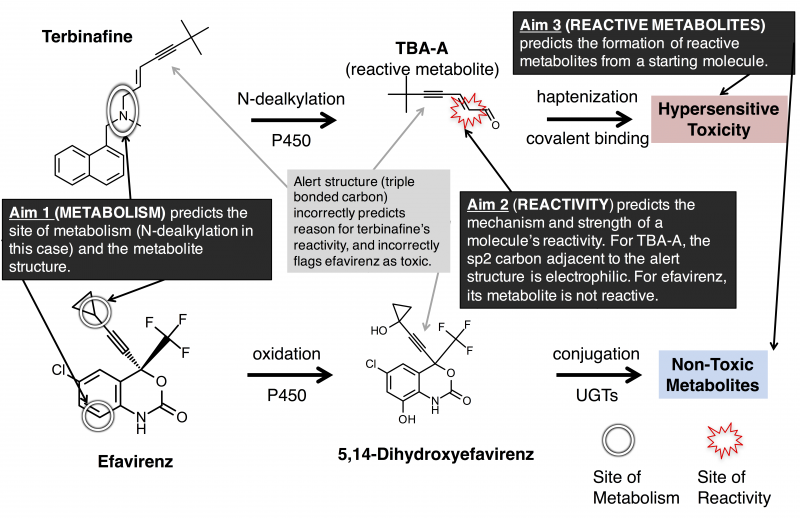
Adverse drug reactions (ADRs) are dangerous and expensive, afflicting about 1.5% of hospitalized patients with profound health and financial consequences. In Medicare patients alone, adverse drug reactions account for 19% of total spending ($339 billion), more than 1,900 deaths, and more than 77,000 extra hospital days per year. Idiosyncratic ADRs, especially rare and severe hypersensitivity-driven ADRs, are the leading cause of medicine withdrawal and termination of clinical development. At the same time, a large proportion of drugs are not associated with hypersensitivity driven ADRs, offering hope that new medicines could avoid them entirely with reliable predictors of risk. Hypersensitivity driven ADRs are caused by the formation of chemically reactive metabolites by metabolic enzymes. These reactive metabolites covalently attach to proteins to become immunogenic and provoke an ADR. Unfortunately, current computational and experimental approaches do not reliably identify drug candidates that form reactive metabolites. These approaches are limited because they inadequately model metabolism, which can both render toxic molecules safe and safe molecules toxic.
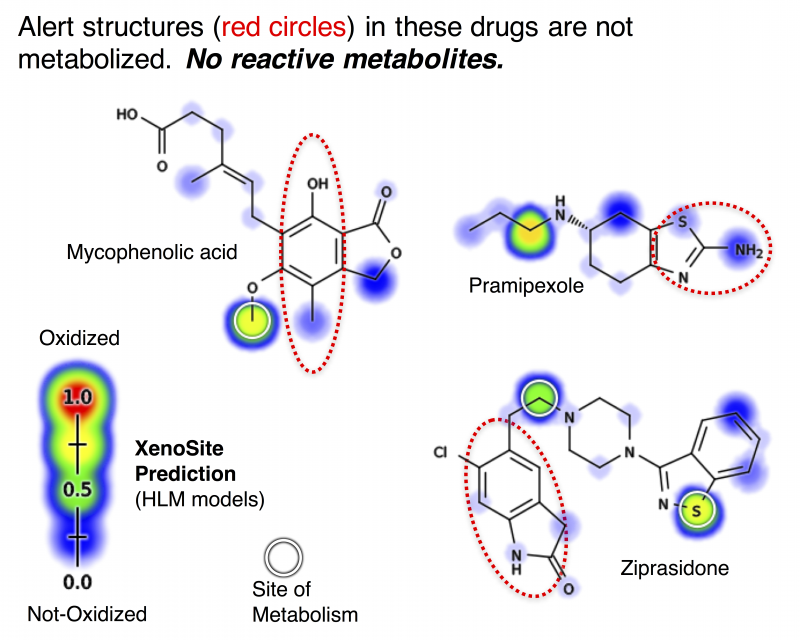
To overcome this limitation, the proposed study aims to curate a public database of metabolism and reactivity and use this database to build accurate and validated mathematical models of metabolism and reactivity. The models will be constructed using machine-learning algorithms that quantitatively summarize the knowledge from thousands of published studies. The computational models generated by these Aims will be validated through statistical approaches and against bench-top experiments. Taken together, this approach will substantially improve on existing approaches by more accurately modeling the properties determining whether metabolism renders drugs toxic or safe.

You must log in to post a comment.